https://www.youtube.com/watch?v=2oPUyIbPxLo&list=PL6CD5AlpaPUH-kF4PwHKAV25scr82dNdq
Introduction to MS
For an unknown compound, the first thing for us is to determine what is the molecular weight of the compound.

We gave the C-H an electon beam, which will knock out a core electron out. Then we will create a molecular ion after we dislodged an electron.
One specie gets the positive charge and the other one gets the electron.
Let the charged ion pass a magnet area, then it will hit at a certain location on the detector.
The case dislodging the core electron

\[r = \frac{mv}{qB}
\]

The case dislodging the bonding electron
We get a carboncation and the hydrogen with extra electron there.
We generate a fragment. But the fragments can be multiple species.


Result
Molecular ion peak + fragment ion peak

The toppest peak is called base peak.


The base peak in the spectrum is usually due to the most stable fragment
Sample Question

Interpreting M+ Peaks in MS
Key points
- The relative intensities on the mass spectrum correspond to the relative abundances of isotopes
\[\begin{equation}
\text { 2. \# of carbon atoms }=\frac{\text { relative intensity of } M+I \text { peak }}{.011 \times(\text { relative intensity of } M \text { peak) }}
\end{equation}
\]
- M+ peaks can indicate if a compound contains an atom(s) pther than carbon

The relatively abundance of C12 and C13 is 98.89% and 1.11%.
This formula works also to other atoms like Br, Cl

Question
Sample problem: The spectrum of a corresponding molecule containing a halogen has the following data:


Relative Abundances

MS Fragmentation
Key Points
- Three general types:
- Heterolytic cleavage
- Homolytic cleavage(alpha)
- McLaferty Rearrangement
- Halogens, ethers and alcohols can cleave heterolytically and homolytically
- Alcohols can fragment by loss of water
- Ketones can fragment homolytically and via McLafferty Rearrangement
Heterolytic cleavage
Alcohol, halogon, ethers


Homolytic Cleavage(alpha cleavage)

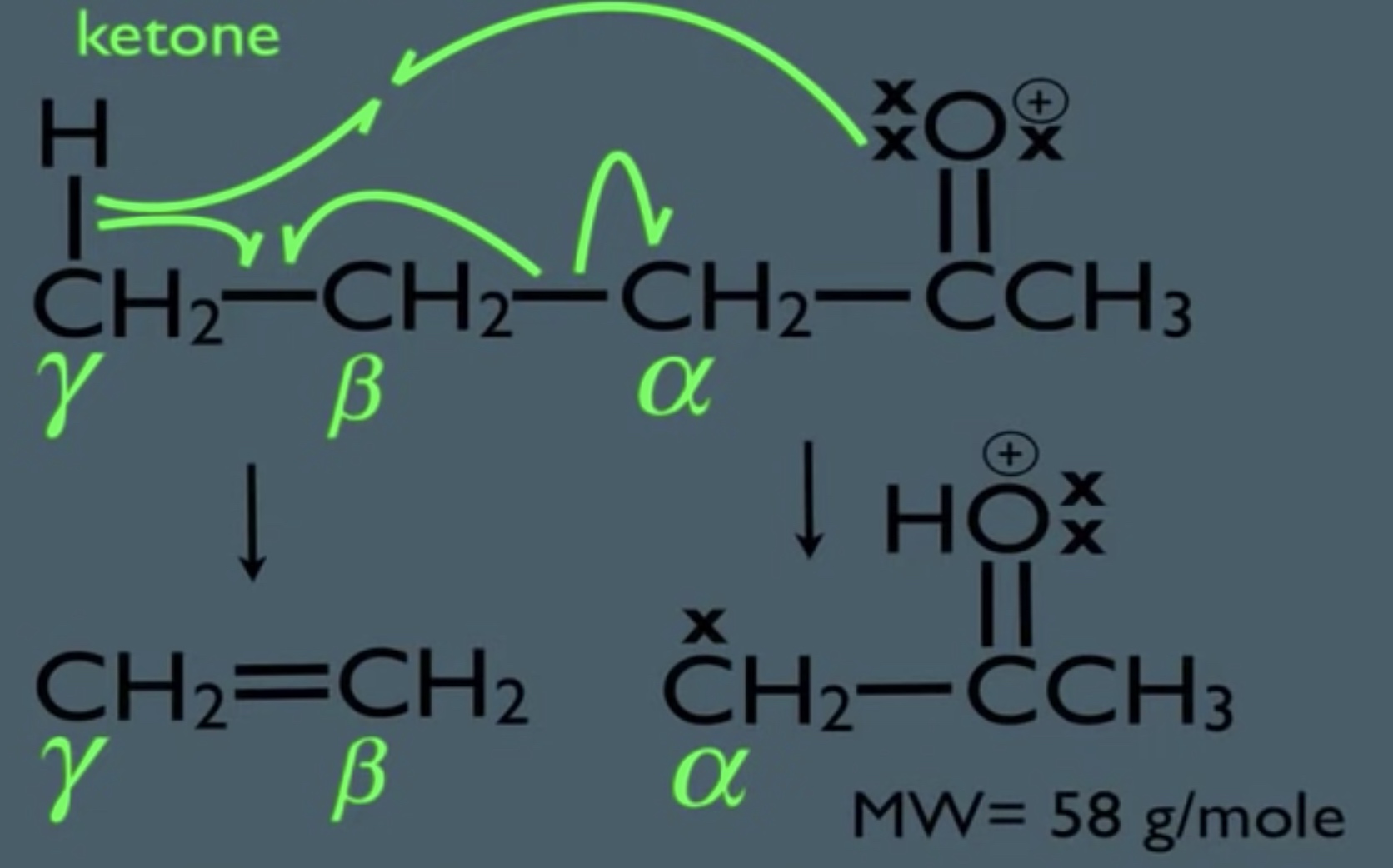
MS Gamma Cleavage Sample Problem


















